Abstract
Background Expression of CD20 antigen is generally negative in the immunophenotype pattern of patients (pts) with Multiple Myeloma (MM). However, a small number of MM pts can show various degrees of CD20 expression on neoplastic plasma cells: this CD20 positivity is described in about 15-20% of pts, with some morphological and clinical peculiar features. However, the clinical and prognostic significance of this CD20 expression is still a matter of debate.
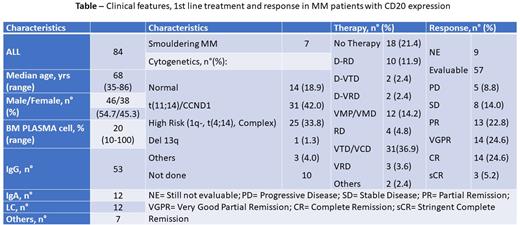
Methods To highlight clinical features and treatment response in this subset, we collected data on 84 pts [46 males and 38 females, median age 68 years (range 35-87)] with MM and positive CD20 expression diagnosed and followed at 6 Italian Centers from 01/2015 to 06/2022. CD20 expression was evaluated with immune-hysto-chemistry or flow-cytometry. Conventional karyotype/FISH on bone marrow plasmacells were performed in all patients: high-risk karyotype was considered in cases with 1q deletion or 17p/p53 or t(4;14) or >/= 3 aberrations (complex karyotype)
Results Clinical features at diagnosis and response to 1st line therapy are reported in the Table1. Karyotype, evaluable in 74 pts, was normal in 14/74 pts (18.9%): among the remaining 60 pts with altered karyotypes, a t(11;14) and/or an involvement of the CCND1 gene (located on the long arm of chromosome 11 - band 11q13) were reported in 31 pts (42.0% of the entire cohort evaluable for karyotype, 51.7% of patients with altered karyotype). Eighteen out of 84 pts (21.4%) were asymptomatic without SLIM-CRAB criteria and did not receive any treatment up to now, while the remaining 66 pts (78.6% of the entire cohort) were symptomatic and received 1st line treatment as reported in the Table 1. Among these 66 treated pts, 57 were already evaluable for response: of them, 17/57 pts (29.8%) had a complete response (CR) (3 sCR and 14 CR), 14 pts (24.6%) a very good partial response, 13/57 pts (22.8%) a partial response and 13/57 pts (22.8%) a stable disease or progression. At the last follow-up, 1 pts died from acute myocardial infarction during Covid-19 infection.
Conclusions In this multicentric real-life cohort of patients with MM and CD20 positivity, high rates of t(11-14)/CCND1 gene alterations were reported: in addition, response to treatment seems worse in comparison with MM pts not expressing CD20, with high rates of stable disease after 1st line therapy. However, larger cohorts of pts are warranted to confirm these results and to evaluate the role of a possible anti-CD20 tailored treatment. Complete data on a longer follow-up will be reported at the meeting.
Disclosures
Fazio:BMS CELGENE: Honoraria; Janseen: Honoraria; gsk: Honoraria. Latagliata:Novartis: Honoraria; BMS: Honoraria. Petrucci:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Celgene/Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Roche: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal